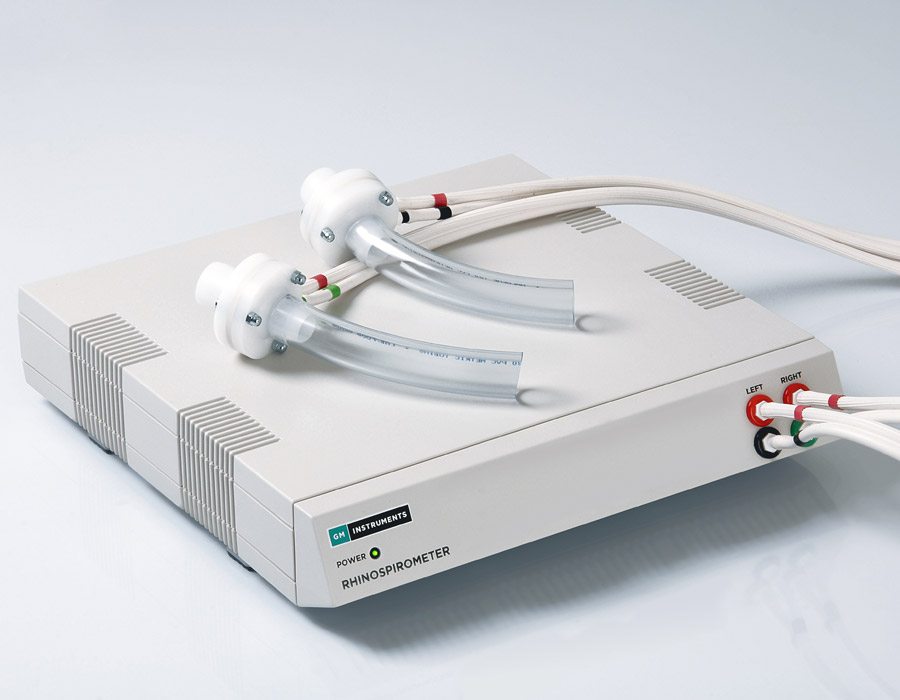
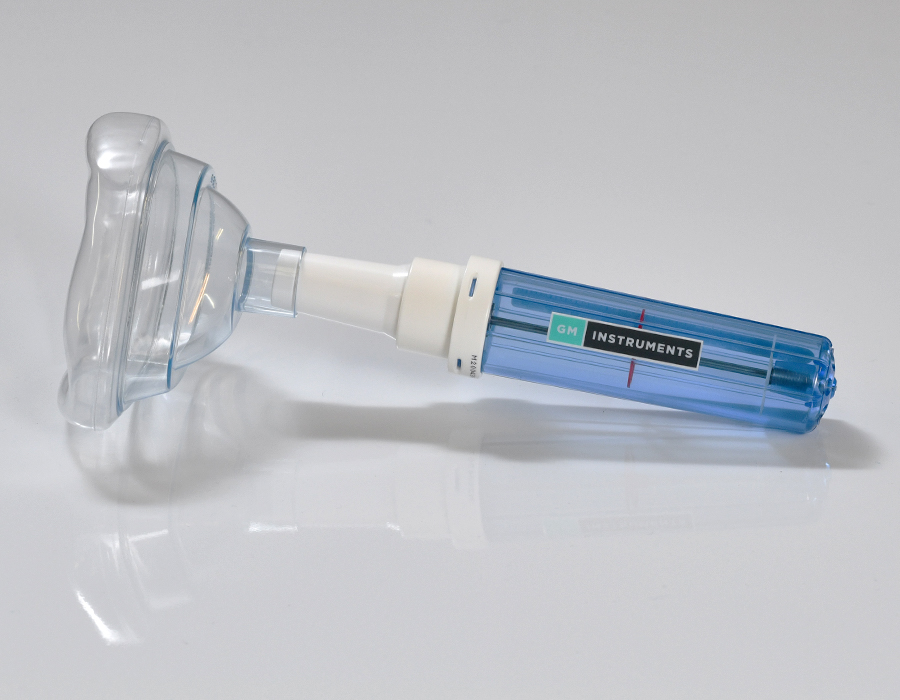
NV2 Rhinospirometer
The NV2 Rhinospirometer is a stand-alone device which allows the measurement of portioning of airflow between two nasal passages and provides a Nasal Partitioning Ratio (NPR).
The NV2 Rhinospirometer has a number of benefits to the user:
- Aids selection of patients for Septoplasty
- Software compatible with Windows 10
- Rapid test which requires minimal patient co-operation
- Provides the user with documented confirmation of the success of the procedure
- Pre/Post challenge or operative comparisons
- Anatomical or conical nose pieces for rapid connection
- Small & lightweight
Used with subjects who have a Septal Deviation, clinical work has identified a key ratio, above which subjects are likely to benefit from surgery and below which, they are unlikely to benefit.
As the patient breathes in through two nasal adaptors, the volume of air through each side of the nose is measured. A nasal partitioning ratio (NPR) can be easily calculated from the nasal volumes.
Clinical work published suggests that NPR values within a range of +/-0.3 are normal while figures greater than that indicate a degree of asymmetry, which may benefit from surgery.
Developed in conjunction with the Common Cold Centre Cardiff University, UK.
| Dimensions | 21x8x15cm |
| Weight | 2kg |
| Supply Voltage | Universal (Free-standing model) / USB (PC Link) |
| Power Consumption | 3 watts |
| Repeatability | > 2% FSD |
| Volume Accuracy (0–5L) | > 3% FSD |
| Standards | BS EN 60601-1: Edition 3.1 plus EMC Edition 4, Class IM, CE Mark |
| Warmup Time | 5 Minutes |
Examples of the NV2 reference papers:
- M. M. Martin. Treatment success after rhinosurgery: an evaluation of subjective and objective parameters. European Archives of Oto-Rhino-Laryngology 2021.
- Cuddihy PJ, Eccles R. The use of nasal spirometry as an objective measure of nasal septal deviation and the effectiveness of septal surgery. Clin Otolaryngol 2003;28(4):325-30.
- Cuddihy PJ, Eccles R. The use of nasal spirometry for the assessment of unilateral nasal obstruction associated with changes in posture in healthy subjects and subjects with upper respiratory tract infection. Clin Otolaryngol 2003;28(2):108-11.
- Hanif J, Eccles R, Jawad S. The use of a portable spirometer for studies on the nasal cycle. American Journal of Rhinology 2001;15:303-306.
- Hanif J, M JSS, R E. Spirometry vs. rhinomanometry for studies on the nasal cycle. Clinical and Experimental Allergy 2001;31(7):28-.
- Hanif J, Jawad SS, Eccles R. A study to assess the usefulness of a portable spirometer to quantify the severity of nasal septal deviation. Rhinology 2003;41(1):11-5.
- Roblin DG, Eccles R. Normal range for nasal partitioning of airflow determined by nasal spirometry in 100 healthy subjects. Am J Rhinol 2003;17(4):179-83.
- Boyce, J.M., & Eccles, R. Assessment of subjective scales for selection of patients for nasal septal surgery. Clin. Otolaryngol. 2006 31, 1-6.
- HIPPOKRATIA 2012, 16, 2: 166-169. Lateralized olfactory.
Sign up for the latest news, events and offers from us:
*By signing up, you are consenting to the HCE Medical Group holding your data and contacting you.